Intraparenchymal cerebral hemorrhage in a patient undergoing treatment with galcanezumab: the importance of adequate blood pressure control
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Accepted: 17 July 2025
Authors
Background: Calcitonin gene-related peptide (CGRP) is a neuropeptide involved in pain transmission and modulation, and implicated in migraine pathophysiology. Due to the vasodilatory action of CGRP, anti-CGRP drugs, while ameliorating migraine, may increase hypertension, a major risk factor for cerebrovascular diseases. Although most studies support the safety of this class of drugs, the use of anti-CGRP drugs in some individuals has been associated with elevated blood pressure.
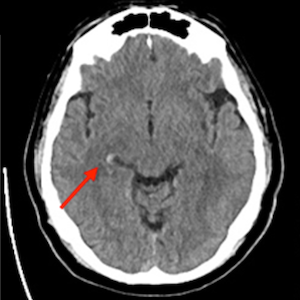
Case Presentation: We report a case of a cerebral hemorrhage in a patient treated with an anti-CGRP monoclonal antibody and a poorly controlled blood pressure.
Discussion: Migraine is associated with increased cerebrovascular risk and hypertension, and anti-CGRP therapies could potentially contribute to acute hypertensive episodes, possibly increasing the risk of complications, including cerebral hemorrhage, in vulnerable individuals.
Conclusions: Limited evidence links anti-CGRP therapies to hypertension. Pending additional data, caution is recommended when prescribing these drugs, especially in patients with cardiovascular risk factors.
How to Cite

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






